About POPPY
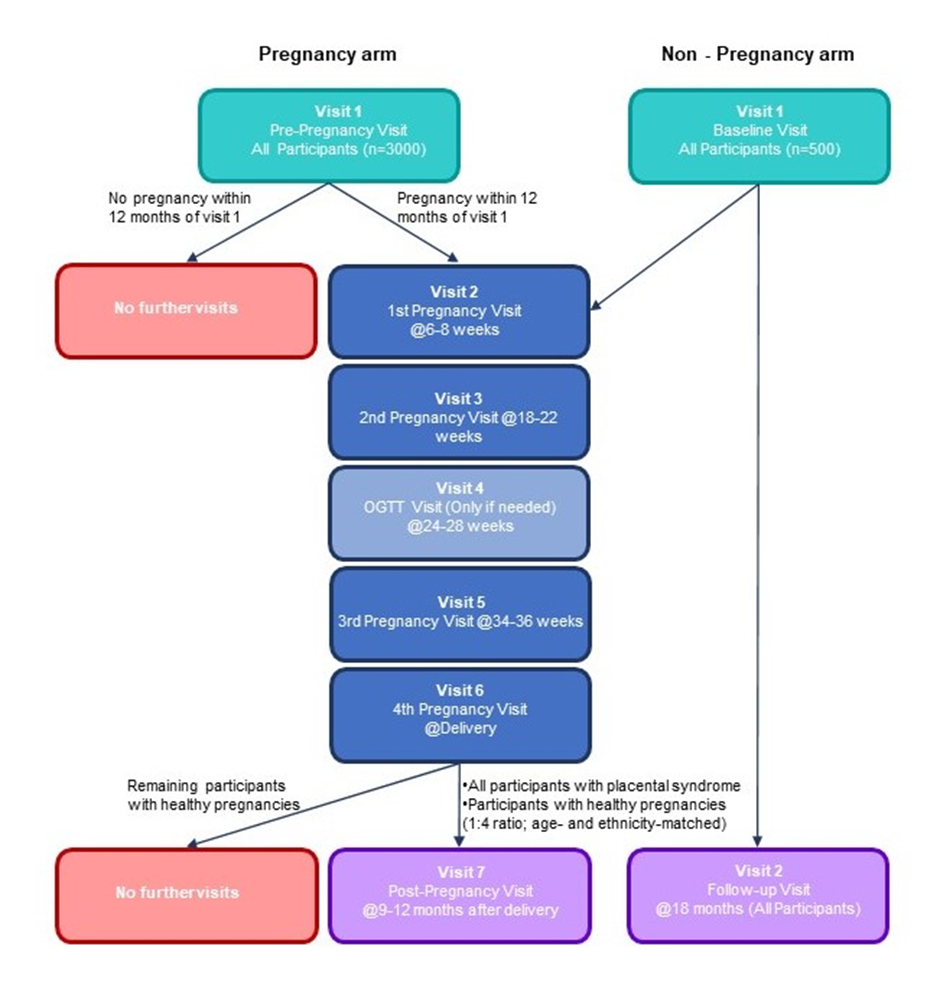
POPPY is the largest study of its kind in the UK. It is an observational, prospective study consisting of two arms: a Pregnancy arm (women planning their first pregnancy) and a Non-Pregnancy arm (women voluntarily planning not to conceive during their involvement in the study).
To learn more about the study, please see below.
For more detailed information about taking part in the Pregnancy Arm please refer to our Pregnancy Arm MINI Participant Information Sheet.
For more detailed information about taking part in the Non-pregnancy Arm please refer to our Non-pregnancy Arm MINI Participant Information Sheet.
Participants in the pregnancy arm will receive pregnancy and ovulation kits!
Exclusion criteria:
We are unable to include anyone who:
- Is unable to give their informed consent
- Is aged less than 18 or greater than 45 years old
- Has been pregnant previously (beyond 20 weeks’ gestation) or is pregnant at the first study visit
- Has established infertility or is using fertility treatments (e.g. IVF, ICSI, FET, IUI)
- Was assigned male sex at birth
- Has an autoimmune disease (e.g. rheumatoid arthritis, lupus)
- Has thrombophilia
- Has type 1 diabetes
- Has advanced chronic kidney disease (stages 4-5)
- Has malignant hypertension
- Has clinically manifest cardiovascular disease (e.g. previous myocardial infarction, stroke)
- Has active cancer/ is being treated for cancer currently (other than skin cancer)
- Has any other condition preventing full participation in the study
POPPY Study Sites
The POPPY study is run across 6 participating sites: Addenbrooke’s Hospital in Cambridge, Glasgow Royal Infirmary in Glasgow, St Mary’s Hospital in Manchester and three hospitals in London: St George’s Hospital, Queen Charlotte’s and Chelsea Hospital and University College Hospital (currently not recruiting).
Please note that recruitment to the non-pregnancy arm is currently paused, and sites are recruiting only to the pregnancy arm.
POPPY Study Visits